Bioinorganic Radiochemistry
Prof. Dr. Thomas L. Mindt
Marker für die Aktivität von Albumin-bindenden Verbindungen
Es gibt mehr und mehr Anhaltspunkte, dass sich Krebszellen in ihren Strategien Nährstoffe aufzunehmen von gesundem Gewebe unterscheiden. Speziell ihre erhöhte Aufnahme von Plasmaproteinen, wie zum Beispiel Albumin, stellt einen vielversprechenden Ansatzpunkt für die Therapie dar. Dies konnte bereits durch den klinischen Erfolg von Medikamenten wie Abraxane (ein Albumin-Paclitaxel Nanopartikel) oder Aldoxorubicin (ein Albumin-bindendes Doxorubicin) unterstrichen wird. Auf Grund dessen ist es von großem Interesse weitere Medikamente zu entwickeln, welche über Albumin spezifisch in die Krebszelle transportiert werden. Zu diesem Zweck haben wir kürzlich die erste Albumin-bindende Prodrug von Oxaliplatin entwickelt. Diese neue Verbindung, welche vielversprechende Aktivität gegen Dickdarmkrebs zeigt, wäre eine gute Möglichkeit das häufig in Kolorektalkrebs verabreichte FOLFOX Schema (Folinsäure, 5-Fluorouracil und Oxaliplatin) zu verbessern. Doch obwohl das Potential der Albumin-vermittelten Therapie für die Behandlung von Krebserkrankungen schon klinisch geprüft wurde, ist das Wissen über die Unter-schiede im Albumin Metabolismus zwischen Krebszellen und gesunden Zellen überraschend gering.
Die Ziele des hier präsentierten Antrags sind einerseits das bessere Verstehen der Albumin Homöostase in Dickdarmkarzinomen und andererseits das Verwenden dieses Wissen für die präklinische Entwicklung einer neuen Albumin-bindenden Oxaliplatin-Prodrug. Ein Schwerpunkt bildet die Rolle potenzieller Biomarker (z.B. K-RAS) in der Albuminaufnahme von malignen Zellen. Dies soll im Rahmen dieses Projektes durch verschiedenen zell-und molekularbiologischer Methoden analysiert werden. Zusätzlich wird eine neue PET Bildgebungsplattform, unter der Verwendung von 89Zr-markierten Albuminkonjugaten, etabliert. Diese soll die Untersuchung der Albuminaufnahme in Tumorknoten in vivo in einer nicht invasiven und quantitativen Art und Weise ermöglichen und so als Hilfsmittel für die Stratifizierung zukünftiger Patientenkohorten herangezogen werden können.
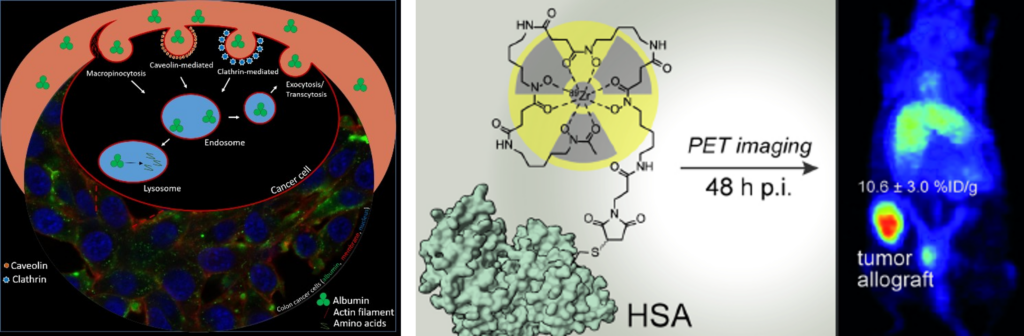
[1] “Site-Selectively Functionalized Albumin with DFO*Maleimide for 89Zr-Radiolabelling Yields a Metabolically Stable PET Probe that Enables Late Time-Point Tumor Imaging in Mice” J. Kronberger, T. Balber, H. Schueffl, R. Wahrmann, A. Federa, M. Gradl, M. R. Brandt, T. Wanek, M. Mitterhauser, C. R. Kowol, T. L. Mindt*, P. Heffeter Journal of Medicinal Chemistry 2025, accepted. DOI: https://doi.org/10.1021/acs.jmedchem.5c00803
Funding
Austrian Science Fund No P 32886-B
Principle Investigator
Prof. Dr. Petra Heffeter (Medical University of Vienna)
Co-Investigators
Prof. Dr. Thomas Mindt (LBIAD), Prof. Dr. Christian Kowol (University of Vienna)
Funding: kEUR 400
Duration: 2020-2024
Radiostar – Novel Chelators for Radio-Lanthanides and -Actinides
Radioactive labelled drugs (radiopharmaceuticals) are used in nuclear medicine for the diagnosis and therapy of cancer. In recent year, new therapeutic radiometals have emerged and showed great potential for targeted tumour therapy. For example, the alpha particle emitting radiometal actinium-225 (Ac-225) is particularly of current interest for endoradiotherapy. Medical applications of radioactive metals require chelators to attach them to tumour-targeting biomolecules (e.g., proteins). Such chelators must form complexes with the radiometal of sufficient stability so that the radioactive label does not dissociate from the bioconjugate in vivo. Suitable chelators are known for a number of radioactive transition metals. However, the size and complexation chemistry is different for the lanthanides and actinides (e.g., Ac-225). As a result, only few examples of chelators for these radiometals have been investigated and no ideal candidate has yet been reported. There is a clear need for new chelators that will facilitate and expedite clinical applications of the emerging and promising radioactive lanthanides and actinides.
Based on previously developed chemistry, novel chelators for lanthanides and actinides will be synthesized and attached to biomolecules. The bioconjugates will be labelled with radiometals and fully evaluated in vitro (e.g. on cells) for their stability and tumour targeting properties (e.g., affinity towards the target). Promising candidates will be selected and used for studies in mice bearing tumour xenografts (biodistribution experiments and small animal PET/CT imaging).
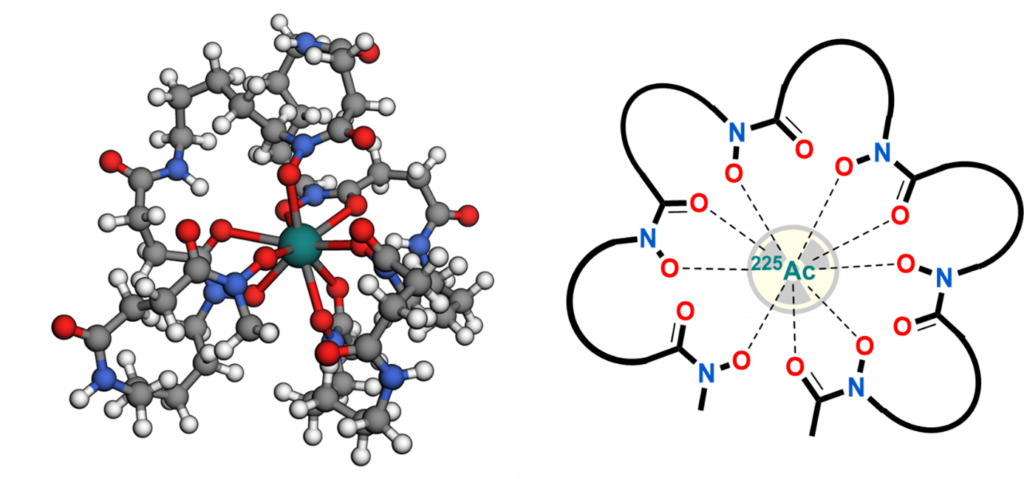
[1] “Towards DFO*12—Preliminary Results of a New Chelator for the Complexation of Actinium-225” I. V. J. Feiner, D. Svatunek, M. Pressler, T. Demuth, X. Guarrochena, J. H. Sterba, S. Dorudi, C. Pichler, C. Denk and T. L. Mindt* Pharmaceutics 2025, 17, 320. DOI: https://doi.org/10.3390/pharmaceutics17030320
Funding
Austrian Research Promotion Agency (FFG), Bridge-1 Project
Principle Investigator
Prof. Dr. Thomas L. Mindt
Co-Investigators
Dr. Christoph Denk (Technical University of Vienna) Clemens Pichler (DSD-Pharma, AT)
Funding: kEUR 450
Duration: 2020-2023
Novel Multidentate Bifunctional Chelating Agents for the Development of Zirconium-89 Based Molecular Imaging Probes
Zirconium-89 (89Zr) based radiotracers hold great promise as immuno-PET imaging agents in nuclear medicine (PET = positron emission tomography). However, insufficient stability of currently used radiometal complexes in vivo is a safety concern for clinical applications. We have developed a novel bifunctional, octadentate chelator (termed DFO*),[1] which provides 89Zr-labelled immuno-conjugates of remarkably improved stability in vitro and in vivo.[2] We are currently investigating further optimization of the new scaffold DFO* and its application for the development of a range of metal-based radiopharmaceuticals.
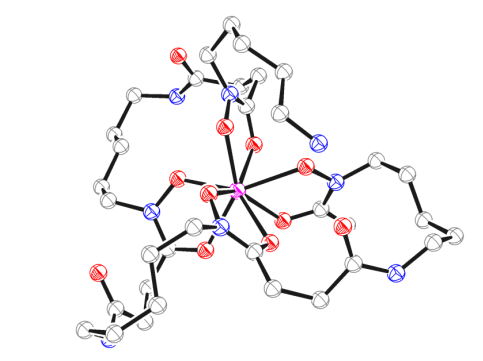
[1] “An Octadentate Bifunctional Chelating Agent for the Development of Stable Zirconium-89 Based Molecular Imaging Probes” M. Patra, A. Bauman, C. Mari, C. A. Fischer, O. Blacque, D. Häussinger, G. Gasser, T. L. Mindt Chemical Communications 2014, 50, 11523-11525.
[2] “Comparison of the Octadentate Bifunctional Chelator DFO*-pPhe-NCS and the Clinically Used Hexadentate Bifunctional Chelator DFO-pPhe-NCS for 89Zr-Immuno-PET” D. J. Vugts, C. Klaver, C. Sewing, A. J. Poot, K. Adamzek, S. Huegli, C. Mari, I. E. Valverde, G. Gasser, T. L. Mindt, G.A.M.S. van Dongen European Journal of Nuclear Medicine and Molecular Imaging 2017, 44, 286-295.
[3] “A Solid Phase-Assisted Approach for the Facile Synthesis of a Highly Water Soluble Octadentate Zirconium-89 Chelator for Radiopharmaceutical Development” M. Briand, M. Aulsebrook, T. L. Mindt, G. Gasser, Dalton Transactions 2017, 46, 6387-1638
Funding
Swiss National Science Foundation Grant N° 205321–157216
Principle Investigator
Prof. Dr. Thomas L. Mindt
Co-Investigator
Prof. Dr. Gilles Gasser (Chimie ParisTech, formerly at University of Zurich)
Funding: kEUR 406
Duration: 2018 – 2023
Radiolabelled Peptides for the Imaging of the PD-L1 Receptor
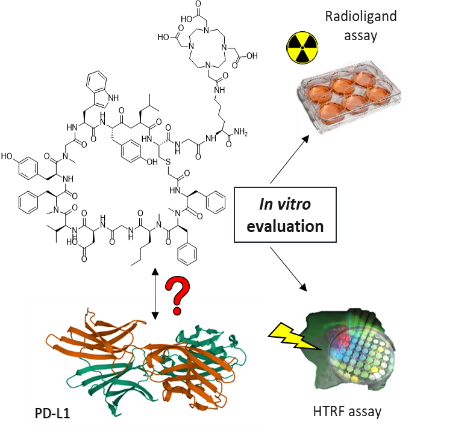
Inhibiting the interaction between the immune checkpoint PD-1 and its ligand PD-L1 activates the immune system to fight cancer. Detection of the expression of PD-L1 is a prerequisite for immune checkpoint inhibition therapy. In this project, we focus our efforts towards the development of a PD-L1 specific nuclear imaging probe based on a peptides.
Selected Publications
“Evaluation of a Macrocyclic Peptide as Potential PET Imaging Probe for PD-L1” N. Jouini, J. Cardinale, T. L. Mindt ChemMedChem2022, 17, E202200091
Funding
Ludwig Boltzmann Society (2018-2021), Austria
Principle Investigator
Prof. Dr. Thomas L. Mindt
Metabolically Stabilized Triazolo-Peptidomimetics for Improved Tumor-Targeting

Labile amide bonds in peptides can be replaced by triazoles to enhance their metabolic stability and tumor uptake in vivo.
A: 1,4-disubstituted 1,2,3-triazoles as metabolically stable bioisosteres of trans amide bonds. B: 1,5-disubstituted 1,2,3-triazoles as metabolically stable bioisosteres of cis amide bonds.
Replacement of amide bonds in the backbone of peptides by 1,2,3-triazole heterocycles increases the metabolic stability and tumor-uptake of peptide-based radiotracers (see also current funded projects).
We have applied this amide-to-triazole switch methodology to several radiolabeled, tumor-targeting peptides including bombesin, neurotensin, minigastrin and other peptides. In all cases, a triazole-scan (systematic replacement of each single amide bond) yielded triazolo-peptidomimetics with improved blood serum stability, maintained biological properties and enhanced tumor-uptake in mice bearing tumor xenografts. In most cases, multiple replacements of amide bonds with triazoles was possible without impairment of the peptide´s biological function.
The amide-to-triazole switch methodology is fully compatible with efficient manual and automated solid phase peptide synthesis.
Selected Publications: –
- “1,2,3-Triazoles as Amide Bond Mimics: Triazole Scan Yields Protease-Resistant Peptidomimetics for Tumor Targeting” I. E. Valverde, A. Bauman, C. A. Kluba, S. Vomstein, M. Walter, T. L. Mindt Angewandte Chemie International Edition, 2013, 52, 8957-8960
- “Probing the Backbone Function of a Bombesin-Based Radiotracer by an Amide-to-Triazole Substitution Strategy” I. E. Valverde, S. Vomstein, T. L. Mindt Journal of Medicinal Chemistry 2015, 58, 7475–7484
- “Radiolabeled Analogs of Neurotensin (8-13) Containing Multiple 1,2,3-Triazoles as Stable Amide Bond Mimics in the Backbone” A. Mascarin, I. E. Valverde, T. L. Mindt Medicinal Chemical Communications 2016, 7, 1640-1646
- “Amide-to-Triazole Switch vs. In Vivo NEP-Inhibition Approaches to Promote Radiopeptide Targeting of GRPR-Positive Tumors” T. Maina, A. Kaloudi, I. E. Valverde, T. L. Mindt, B. A. Nock Nuclear Medicine and Biology 2017, 52, 57-62
- “Design of Radiolabeled Minigastrin Analogs by Multiple Amide-to-Triazole Substitutions for Enhanced Tumor Targeting” N. M. Grob, S. Schmid, R. Schibli, M. Béhé, T. L. Mindt Journal of Medicinal Chemistry 2020, 63, 4496-4505
- “1,4-Disubstituted 1,2,3-Triazoles as Amide Bond Surrogate for the Stabilisation of Linear Peptides with Biological Activity” L. M. Recnik, W. Kandioller, T. L. Mindt Molecules 2020, 25, 3576-3602
- “Single Peptide Backbone Surrogate Mutations to Regulate GPCR Subtype Selectivity” E. I. Vrettos, I. E. Valverde, A. Mascarin, P. N. Pallier, M. Fragai, G. Parigi, B. Hirmiz, N. Bekas, N. M. Grob, E. Stylos, M. Del Borgo, M.-I. Aguilar, F. Magnani, N. Syed, T. Crook, E. Waqif, E. Ghazaly, R. E. Widdop, C. Luchinat, A. T. Michael-Titus, T. L. Mindt, A. G. Tzakos Chemistry – A European Journal 2020, 26, 10690
Funding
– Swiss National Science Foundation (2011-2019), grant No 205321_132280/1 and 2021_157076/1
– Austrian Science Fund (2018-2023) grant No P31477-B28
Principle Investigator
Prof. Dr. Thomas L. Mindt
Click-to-Chelate: An Efficient Approach for 99mTc-Radiolabeling and the Preparation of Multifunctional Radioconjugates

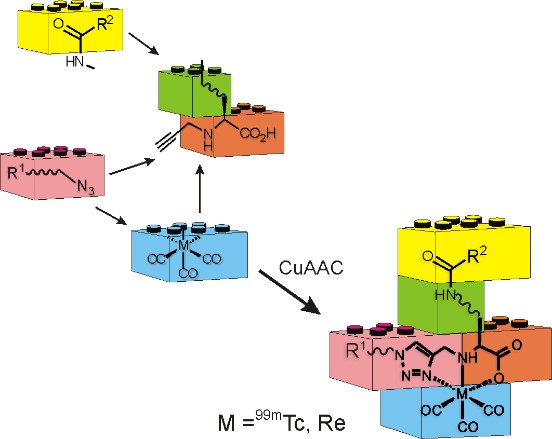
The click-to-chelate approach provides an efficient and versatile strategy for the radiolabeling of biomolecules with the 99mTc-tricarbonyl core as well as other radionuclides. In addition, this methodology utilizes orthogonal chemistry that enable the rapid and straight-forward assembly of multifunctional radioconjugates for various applications.
Selected Publications
- “Click to Chelate: Synthesis and Installation of Metal Chelates into Biomolecules in a Single Step” T. L. Mindt, H. Struthers, L. Brans, T. Anguelov, C. Schweinsberg, V. Maes, D. Tourwé, and R. Schibli Journal of the American Chemical Society 2006, 128, 15096
- “Click Chemistry Radiosynthesis and Preclinical Evaluation of a New 18F-Labelled Folic Acid Derivative” T. L. Ross, M. Honer, P. Y. H. Lam, T. L. Mindt, V. Groehn, R. Schibli, P. A. Schubiger, S. M. Ametamey Bioconjugate Chemistry 2008, 19, 2462
- “A Click Approach to Structurally Diverse Conjugates Containing a Central Di-1,2,3-Triazole Metal Chelate” T. L. Mindt, C. Schweinsberg, L. Brans, A. Hagenbach, U. Abram, D. Tourwé, E. Garcia-Garayoa,R. Schibli ChemMedChem 2009, 4, 529
- “Molecular Assembly of Multifunctional Tc-99m Radiopharmaceuticals using “Clickable” Amino Acid Derivatives“ T. L. Mindt, H. Struthers, B. Spingler, L. Brans, D. Tourwe, E. Garcia-Garayoa, R. Schibli ChemMedChem 2010, 5, 2026-2038
- „Imaging of Activated Macrophages in Experimental Osteoarthritis Using Folate Targeted Animal SPECT/CT“ T. M. Piscaer, C. Müller, T. L. Mindt, E. Lubberts,J. A. N. Verhaar,E. P. Krenning, R. Schibli,M. De Jong, H. Weinans Arthritis & Rheumatism 2011, 63, 1898–1907
- “Click-to-Chelate: Development of Technetium- and Rhenium-Tricarbonyl Labeled Radiopharmaceuticals” C. A. Kluba, T. L. Mindt Molecules 2013, 18, 3206-3226
- “A Bombesin-Shepherdin Radioconjugate Designed for Combined Extra- and Intracellular Targeting” C. A. Fischer, S. Vomstein, T. L. Mindt Pharmaceuticals 2014, 7, 662-675
- “Sorbitol as a Polar Pharmacological Modifier to Enhance the Hydrophilicity of 99mTc-Tricarbonyl-Based Radiopharmaceuticals” C. Giammei, T. Balber, K. Bencurova, J. Cardinale, N. Berroterán-Infante, M. Brandt, N. Jouini, M. Hacker, M. Mitterhauser and T. L. Mindt Molecules 2020, 25, 2680-2694
Funding
ETH Zurich (2005-2009) and University of Basel (2009-2014), Switzerland
Principle Investigator
Prof. Dr. Thomas L. Mindt




