Bioinorganic Radiochemistry
Prof. Dr. Thomas L. Mindt
Metabolically Stabilized Peptidomimetics for Improved Tumour Targeting
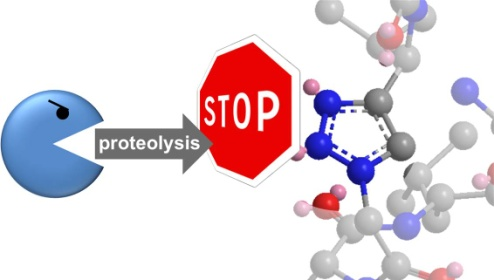
Regulatory peptides are a class of biomolecules with ideal characteristics for the development of tumour-targeting radiopharmaceuticals. They exhibit a high, specific accumulation in tumours but not in healthy tissue and a favourable pharmacokinetic profile. A drawback of using such peptides for the selective delivery of attached radionuclides to tumours is their low stability due to rapid degradation by enzymes (proteases) before they can reach their target (tumours). It is known from the literature that enhancing the metabolic stability of a peptide carrier can substantially increase its uptake in tumours and metastases. Despite considerable research efforts directed towards the stabilization of the peptides without influencing their favourable biological characteristics, no general approach has yet been identified. We have recently introduced a novel “click chemistry” methodology to achieve this goal. We use metabolically stable 1,2,3-triazole heterocycles as biosiosteres of labile amide bonds of the peptides. We were able to show that the obtained, radiolabelled peptidomimetics exhibit an increased stability and, as a result, a significantly improved tumour uptake in mice.
Selected publications:
[1] “1,2,3-Triazoles as Amide Bond Mimics: Triazole Scan Yields Protease-Resistant Peptidomimetics for Tumor Targeting” I. E. Valverde, A. Bauman, C. A. Kluba, S. Vomstein, M. Walter, T. L. Mindt Angewandte Chemie International Edition 2013, 52, 8957-8960.
[2] “1,2,3-Triazole Stabilized Neurotensin-Based Radiopeptidomimetics for Improved Tumor Targeting” A. Mascarin, I. E. Valverde, S. Vomstein, T. L. Mindt Bioconjugate Chemistry 2015; 26, 2143–2152.
[3] “Design of Radiolabeled Minigastrin Analogs by Multiple Amide-to-Triazole Substitutions for Enhanced Tumor Targeting” N. M. Grob, S. Schmid, R. Schibli, M. Béhé, T. L. Mindt Journal of Medicinal Chemistry 2020, 63, 4496-4505.
[4] “1,4-Disubstituted 1,2,3-Triazoles as Amide Bond Surrogate for the Stabilisation of Linear Peptides with Biological Activity” L. M. Recnik, W. Kandioller, T. L. Mindt Molecules 2020, 25, 3576-3602.
[5] “1,5-Disubstituted 1,2,3-Triazoles as Amide Bond Isosteres Yield Novel Tumor-Targeting Minigastrin Analogs” N. M. Grob, R. Schibli, M. Behe, I. E. Valverde, T. L. Mindt Medicinal Chemistry Letters 2021, 12, 585-592.
Funding
Austrian Science Fund Grant N° P 31477-B28
Previous grants (2011-2019): Swiss National Science Foundation Grants No 205321_132280, 2021_157076
Principle Investigator
Prof. Dr. Thomas L. Mindt
Funding: total >kEUR 1100
Duration: 2011-present
Theranostic Peptide Radiotracers in Breast Cancer
Breast cancer impacts one in eight women globally, resulting in over half a million deaths each year. Current diagnostic methods fall short, with ~40% of breast cancers having already spread at the time of diagnosis and mammography often producing high rates of false positives. Additionally, there is a lack of reliable tools for monitoring treatment responses, tracking recurrences, or providing real-time tumour visualisation to aid surgical tumour removal.
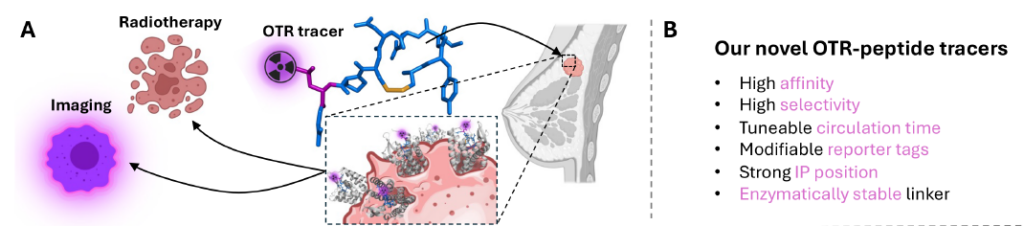
This project addresses these challenges by developing theranostic peptide tracers targeting the oxytocin receptor (OTR), a promising new target for imaging, diagnosis and treatment of breast cancer. OTR is highly overexpressed
in primary breast cancers, including metastatic and triple-negative cases, which remain difficult to treat. Our team has successfully developed potent, OTRspecific peptide tracers capable of visualising OTR in cells and tissues.
Building on this progress, the project will refine and expand the design of these tracers and provide proof-of-concept data that they can be used to efficiently visualise and remove breast tumours. Expected outcomes include theranostic tracers that can enhance breast tumour detection, support breast-conserving surgical tumour removal, and targeted
radiotherapy, including subtypes that currently do not have targeted approaches. This combined approach of improved detection and targeted therapy has the scope to considerably enhance patient care, treatment options, and survival rates while reducing healthcare costs. The development of these tracers, supported by preclinical data and intellectual property protection, is expected to attract further investments and foster industry partnerships to accelerate clinical translation.
Funding
European Research Council (ERC) Proof of Concept (PoC) grant Nr.
Principle Investigator
Prof. Dr. Markus Muttenthaler (University of Vienna)
Co-Investigator
Prof. Dr. Thomas Mindt (University of Vienna), Prof. Dr. Cecile Philippe (Medical University Vienna)
Funding: kEUR 150
Duration: 2024-2025
Development of Novel Chelators for Applications of Emerging Radiometals in Nuclear Medicine for the Diagnosis and Therapy of Diseases
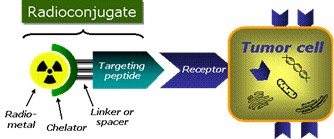
Nuclear medicine focuses on the application of radiolabeled drugs (radiopharmaceuticals, radiotracers) for diagnosis (imaging) or radioendotherapy (internal radiation) of diseases. A variety of different radioiotopes can be used for radiolabeling, but in particular radiometals have shown to be highly suitable for targeted applications. A schematic presentation of a radiometal-based targeted radiopharmaceutical is shown in Figure 1. Molecular nuclear medicine holds the unique potential of being able to locate, diagnose and treat diseases as well as monitor treatment response noninvasively. Targeted molecules equipped with a diagnostic radioisotope deliver the radioactive isotope to the site of the disease where it can be detected by the extremely sensitive nuclear imaging cameras (Table 1). Gamma emitters are used for single‐photon emission computed tomography (SPECT) and positron emitters for positron emission tomography (PET). Using alpha‐ or beta‐particle emitters enables therapeutic applications as their high energy ionizing radiation is cytotoxic by, for example, causing DNA double strand breaks resulting in apoptosis. By an appropriate design of the radiopharmaceutical, e.g., the correct choice of the chelator, radioactive metals of similar chemical properties can be exchanged (for example 68Ga ↔ 177Lu) and thus, provide the possibility of the development of both diagnostic imaging probes and therapeutic agents based on the same targeted conjugate (theranostic approaches).
At the very heart of a radiometal-based radiopharmaceutical lies the chelator. The chelator serves two functions: Firstly, in connects the biological (e.g. tumour-targeting) vector with the radiometal and secondly, it binds the radiometal with sufficient thermodynamic and kinetic stability that enables biological and therefore medical applications in vivo. A plethora of chelators for commonly used radiometals can be found in the literature, however, for new and emerging metallic radionuclides (e.g., Cu-64/67, Zr-89, Sc-44, Tb-161, Ac-225) there is a pressing need for novel suitable chelators in order to enable their use for medical applications in nuclear medicine to the benefit of patients suffering from diseases such as cancer.
Funding
ASEA UNINET
Principle Investigators
Prof. Dr. Thomas Mindt (mentor), Kanyapat Lumjong (Ernst Mach Fellowship)
Funding: kEUR 200
Duration: 2024-2028
Improved Nuclear Imaging by Pretargeting with Small Molecules
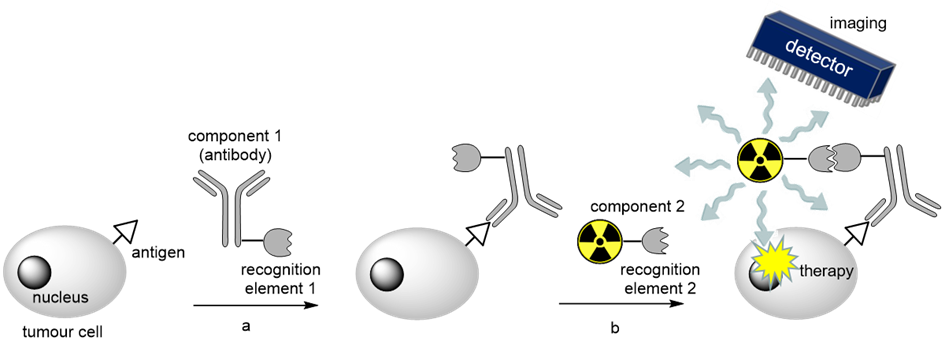
Recently developed pretargeting strategies (PTS) have been shown advantageous for the diagnosis (imaging) of cancer in nuclear medicine. Instead of using directly radiolabeled antibodies (Abs), PTS separate the application of the tumor-targeting biological vector from the radioactive reporter probe and assembles them in vivo at the site of the disease by highly selective and biorthogonal reactions. In brief, a tumor-targeting Ab functionalized with a trans cyclooctene moiety is injected first. After its accumulation at the tumor and clearance from the blood, a radiolabeled tetrazine conjugate is applied. Through the efficient reaction of the two functionalities in vivo, the radiotracer accumulates specifically at tumors while excess is quickly cleared by renal excretion. Thus, PTS reduce the radiation dose to nontargeted tissue as the long blood circulation time of directly radiolabelled Abs is avoided. It also enables the combination of Abs exhibiting long biological half lifes with short lived radionuclides.
PTS in nuclear medicine focus on tumor-targeting Abs. It is proposed to extend this strategy to slowly or only partially cell-internalizing tumor-targeting peptides. In comparison to Abs, the use of peptides brings several advantages including easier accessibility, lower costs, quicker&more efficient tumor targeting and faster clearance from the blood as the results of faster pharmacokinetics. The latter will enable single sessions for a multi-step clinical protocol which increases the patient´s comfort by reducing the number of hospitalizations.
Funding
Austrian Science Fund (FWF) grant Nr. P 36706-B
Principle Investigators
Prof. Dr. Thomas Mindt (University of Vienna), Prof. Dr. Hannes Mikula (Technical University of Vienna)
Funding: kEUR 290
Duration: 2023-2027
Bi-Metallic Complexes for Synergistic and Theranostic Applications
The use of metals in medicine is very versatile, ranging from different diagnostic to therapeutic applications. This project aims at the combination of two metal-based therapies in order to increase the therapeutic efficacy in comparison to the individual treatments alone. Namely, we will combine photodynamic therapy (PDT) with nuclear radioendotherapy (RET). For PDT, metal-containing compounds are excited by light and subsequently, because in aqueous biological media, reactive oxygen species are produced that destroy the surrounding diseased tissue (tumors, infections (viruses) etc.). RET utilizes radioactive metals whose ionizing radiation is toxic and also destroys the surrounding diseased tissue. Goal of this project is to chemically combine two suitable metals (ruthenium and rhenium) in one construct that allows for simultaneous PDT and RET. The bi-metallic compounds will be conjugated to a targeted biomolecule (e.g., antibody) that serves as carrier for the selective transport of the therapeutic cargo to the diseased site while sparing healthy tissue. The bi-metallic compounds and conjugates thereof will be tested biologically (e.g., on cells) in order to investigate the interplay of the two therapies.

[1] “Theranostics with Photodynamic Therapy for Personalized Medicine: To See and to Treat” Y. Wang, J. N. Staudinger, T. L. Mindt*, G. Gasser* Theranostics 2023, 13, 5501-5504. DOI: 10.7150/thno.87363
Funding
Austrian Science Fund (FWF, AT), Agence Nationale de la Recherche (FR) No I 5721
Principle Investigators
Prof. Dr. Thomas Mindt (University of Vienna; AT), Prof. Dr. Gilles Gasser (Chimie ParisTech; FR)
Funding: kEUR 450
Duration: 2021-2026




